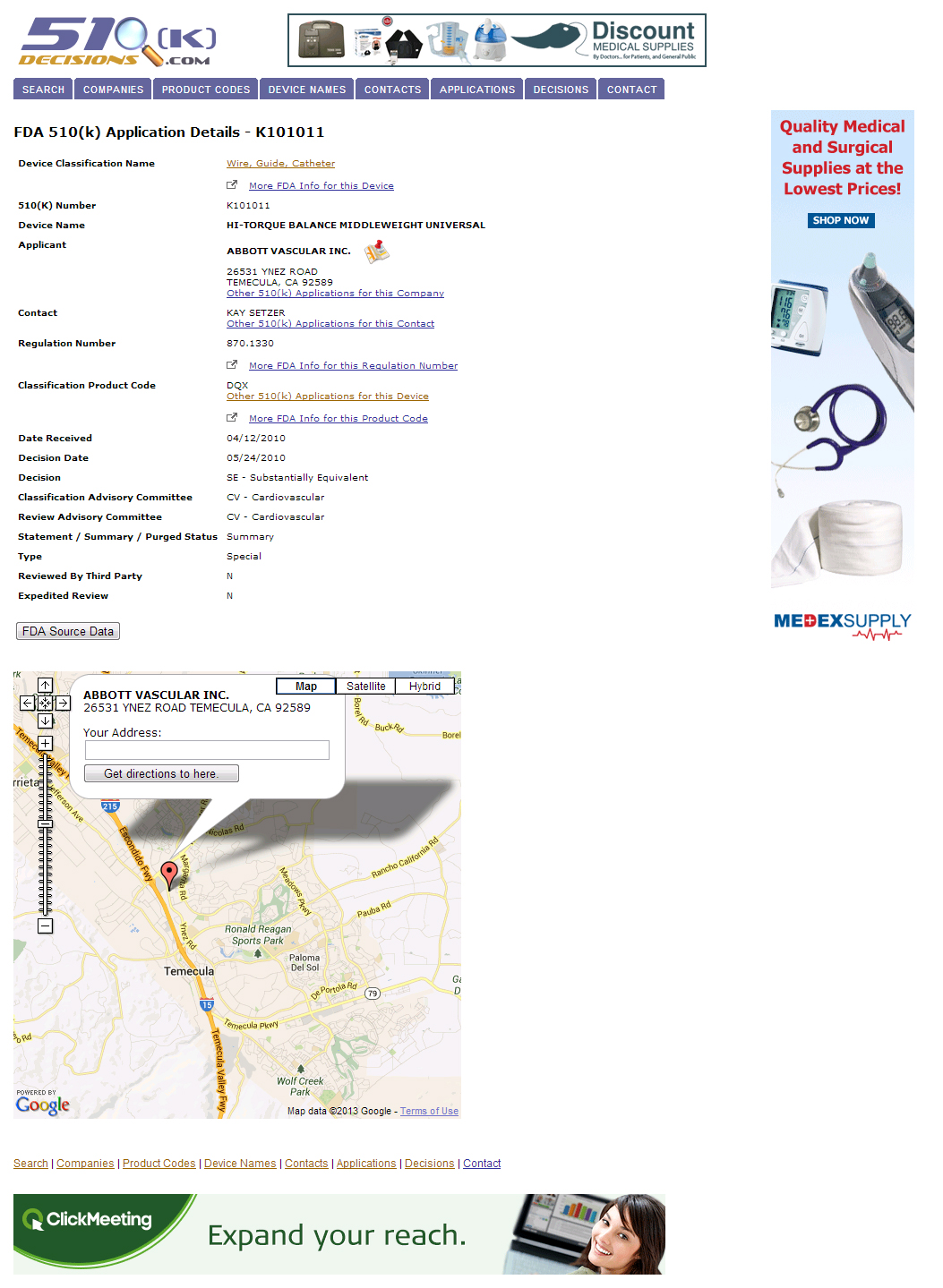
The 510(k) Decisions website contains FDA 510(k) applications and decisions collected since May, 1976 from the U.S. Food and Drug Administration’s publicly available databases of FDA Device Approvals and Clearances. Additionally, it provides convenient cross-linking of records by applicant company name, contact name, device classification, and product code.
I created this directory with ColdFusion and MySQL.
See it live at http://510ks.warrick.net/